American institution includes Pakistan in list of countries working on coronavirus cure
A 20-member team from Abbottabad's Ayub Medical College will work on deriving the cure
April 08, 2020
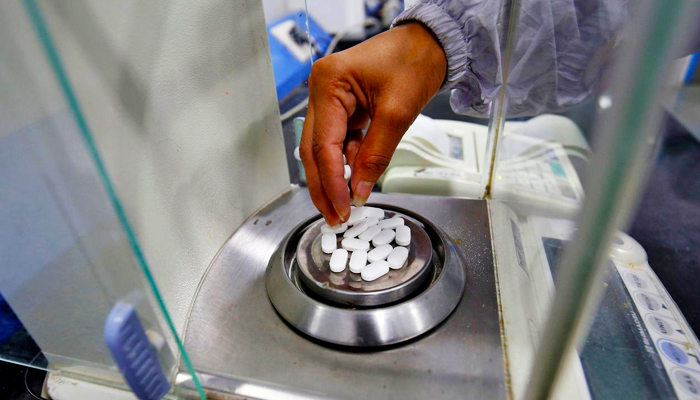
America's National Library of Medicine (NLM) has included Pakistan in the countries to formulate a cure for the coronavirus pandemic, CEO Ayub Medical College Dr Umer Farooq informed Geo News on Wednesday.
Ayub Medical College is a leading public medical institute located in Abbottabad.
The US institution has approved a 20-member team from the medical facility to work on deriving the cure, Dr Farooq said.
"They [NLM] praised Pakistani experts and based on our credentials we were allowed to start testing for the cure," he said, adding: "Groups of 25 people will be made initially for the trials."
"The first group will be given two medicines, azithromycin and chloroquine," he said, adding that the second batch would be given chloroquine only.
Read more: What is chloroquine and could it cure the coronavirus?
"The third group will be treated with traditional medicines," he noted.
People will be selected on the basis of their age and the severity of their diseases, he said. "The ones suffering from heart and major diseases will not be selected for the trial."
"After US' Food and Drug Administration (FDA) approves it, we can hope for a cure for the coronavirus to be available for the world," he added.
Last month, FDA auhtorised two antimalarial drugs touted as game-changers by President Donald Trump.
In a statement, the US Department of Health and Human Services had detailed recent donations of medicine to a national stockpile – including chloroquine and hydroxychloroquine, both being investigated as potential COVID-19 treatments.
It said the FDA had allowed them "to be distributed and prescribed by doctors to hospitalised teen and adult patients with COVID-19, as appropriate, when a clinical trial is not available or feasible."
Trump said last week that the two drugs could be a "gift from God," despite scientists warning against the dangers of overhyping unproven treatments.
Read more: Sanofi, Regeneron expand testing of potential coronavirus treatment
Many researchers including Anthony Fauci, the United States' leading infectious disease expert, have urged the public to remain cautious until larger clinical trials validate smaller studies.
Two US medical bodies – the NIH and the Biomedical Advanced Research and Development Authority – are currently working to plan such trials.
Some in the scientific community fear Trump's endorsement of the medicines could create shortages for patients who need them to treat lupus and rheumatoid arthritis, diseases for which they are approved.