No 'silver bullet': WHO dispels hype around top obesity drugs in market
Wegovy and Mounjaro were originally developed for type 2 diabetes to help control blood glucose
May 13, 2023
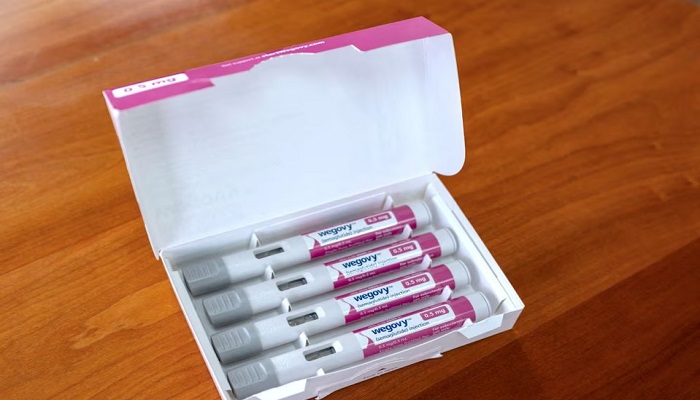
World Health Organization (WHO) Saturday warned against the hype that new weight loss drugs such as Novo Nordisk's Wegovy could prove to be a panacea to the ‘pandemic’ of obesity around the world.
“These drugs are not a “silver bullet” for addressing the rapid rise in global obesity rates,” Francesco Branca, the WHO’s director of nutrition told Reuters, as the UN agency reviews its obesity management guidelines for the first time in over 20 years.
WHO is first brushing up on guidelines for treating children and adolescents with obesity, and will then update its recommendations for adults, said, the WHO director of nutrition and food safety.
The WHO last issued global guidelines on the topic in 2000, which are used as a blueprint for countries without the resources to draft their own plans.
As part of the work, the WHO has commissioned the Mario Negri Institute for Pharmacological Research, in Milan, Italy, to assess the evidence for the use of all drugs for children and adolescents – from older options like GSK's Xenical to newer, more effective treatments like Wegovy and Eli Lilly and Co’s Mounjaro, Branca told Reuters.
“The kind of communication that has been done around these drugs – ‘We’ve found a solution’ – that’s wrong,” said Branca. Drugs for obesity are important but must be “part of a comprehensive approach,” he said. “This is not a silver bullet.”
Branca said that other interventions, including diet and exercise, remain critical to help manage obesity. The latest WHO data shows that the percentage of children and adolescents aged 5 to 19 who are obese or overweight has risen to just over 18% in 2016 from 4% in 1975, and this now represents more than 340 million people.
Novo Nordisk and Eli Lilly did not immediately respond to a Reuters request for comment.
Wegovy and Mounjaro were originally developed for type 2 diabetes to help control blood glucose. More recently, they have been shown to help people lose around 15% of their body weight, capturing the attention of patients, investors and even celebrities.
Part of a class of drugs known as GLP-1 agonists, they are given by a weekly injection and work by affecting hunger signals to the brain and slowing the rate at which a person’s stomach empties, making them feel full longer.
Studies suggest people are likely to have to take the drugs for the rest of their lives to keep the weight off.
Wegovy is approved for weight-loss in the United States and Europe, while Mounjaro is expected to receive U.S. approval later this year. The enormous demand for the drugs is expected to be worth $100 billion in annual sales within a decade, with as many as 10 different drugs on the market.
U.S. medical groups are also reviewing their obesity treatment guidelines to consider the best use of Wegovy and similar drugs, with some specialists advocating broad use while others recommend prioritising them for high-risk patients with health conditions, like diabetes or heart disease, that are exacerbated by excess weight.
The American Academy of Pediatrics has recommended using such medicines in children age 12 or older with obesity, even though the long-term impacts have yet to be studied.
The WHO said its revised guidelines will be based on more robust methodology than previous iterations and include up-to-the-minute science. The first draft of the new management guidelines for children and adolescents are expected by the end of this year.
Branca said the researchers at Mario Negri, as well as other institutions working on the guidelines, had been extensively vetted to avoid conflict of interest concerns.
Novo Nordisk was suspended earlier this year from the Association of the British Pharmaceutical Industry for its marketing practices, such alleged funding of health professionals and providing training that the association alleged was intended to promote its drug.
“We really screen the potential conflicts of interest,” said Branca.
He described obesity as a "rising epidemic".
"There are multiple reasons why we really have to take much more serious and bolder action,” he said.